Applications
Electroplating
What is electroplating?
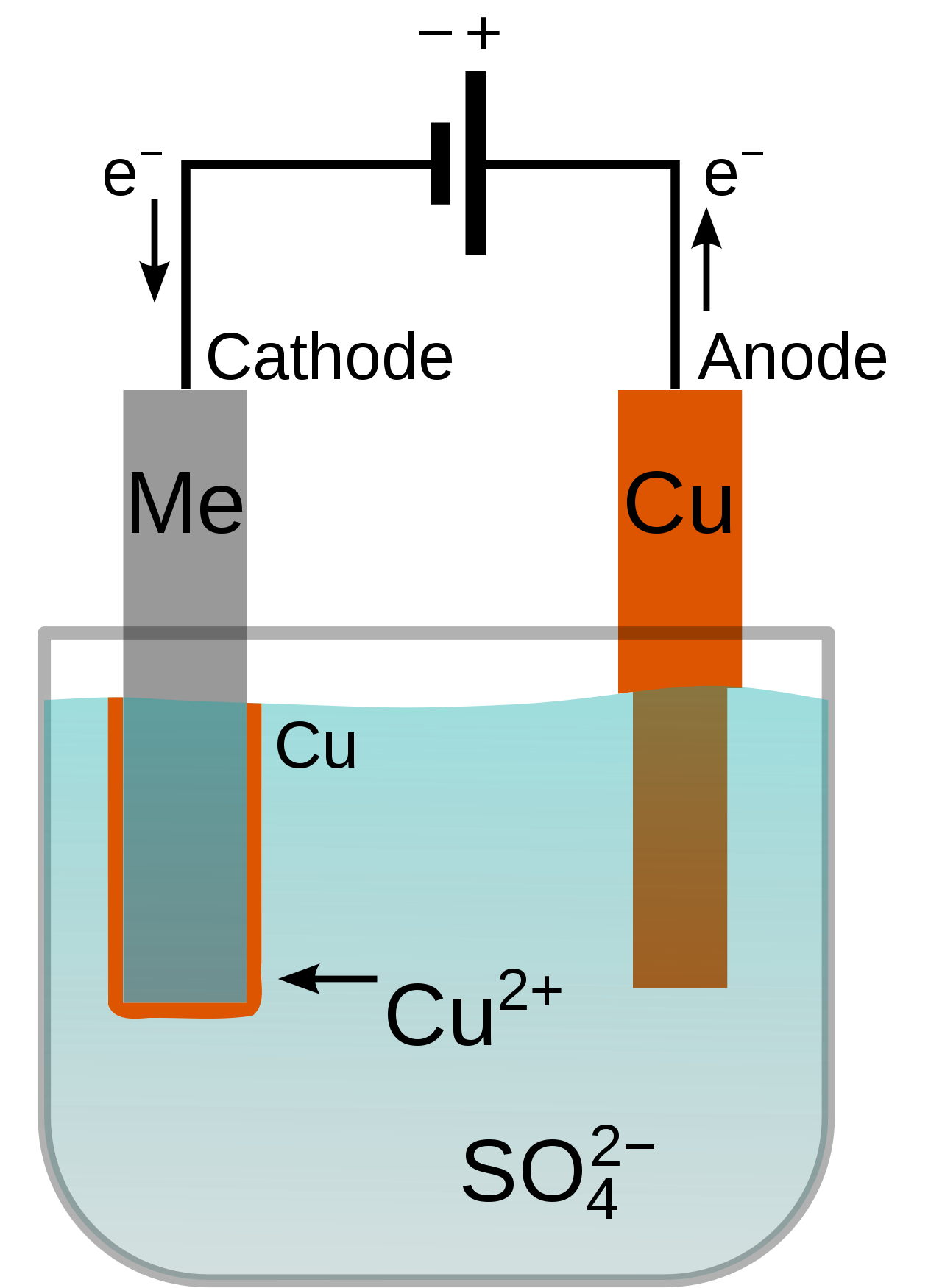
Electroplating is a process that creates a thin metal coating through the means of a direct electrical current. As the diagram above demonstrates, electroplating usually requires the object to be immersed in liquid, but with the Micro-Metallizer Plating Pen, it's as easy as changing the cap on a pen.
Depending what metal you choose, many applications can benefit from an electroplated tool.
Benefits include:
- Increased wear resistance
- Protection against surface abrasions
- Reduced friction
- Improved electrical conductivity
- Prepared surfaces for better adhesion before painting or re-coating
Take a closer look at the electroplating metals we supply and the added advantages they bring.
A short guide to electroplating metals
Tin. Most often used to prevent rusting and corrosion, tin will give your project a semi-matte appearance. It’s commonly used at the ends of wires as an electrical conductor and keeps them from fraying and short-circuiting electronics.
Nickel. With its high-luster finish, nickel is a popular choice for adding a flash of brightness on bumpers, exhaust pipes, trim and rims in the automotive industry. It also provides corrosion and wear resistance.
Black nickel. In contrast to its brighter cousin, black nickel features a low luster shine and is most often used for decorative or military purposes.
Copper. Highly conductive, copper is most often used in semiconductors, circuit boards and other electronic components. Copper is applied to surfaces before other metals, such as nickel and chrome.
Gold. Its aesthetic beauty aside, gold can significantly increase your project’s resistance to tarnishing and corrosion. Apart from being used in fine jewelry, electronics also make use of gold’s conductive properties.
Silver. With a high electric and thermal conductivity, silver is more than just metal for jewelry. Holding a hardness advantage over gold, silver-plating also increases your project’s lifespan.
Chrome. Highly reflective, electroplating with chrome makes your project more resistant to corrosion and makes it easier to clean.
Rhodium. Used almost exclusively for jewelry, rhodium is a highly reflective, hard white metal that adds a protective coat to softer metals. Rhodium is extremely hard to find in nature and is one of the few “white” metals that does not tarnish.
Zinc. Electroplating with zinc is also called “galvanization.” Zinc protects the underlying metal by corroding before the other metal can, protecting the metal as long as zinc is close enough to be electrically coupled. It features a matte finish.
Palladium. Often used as a substitute for gold-plating, palladium has the added benefit of absorbing hydrogen, useful for avoiding hydrogen embrittlement. This metal can protect against corrosion and wear. You will often see palladium in automotive, medical and electronic applications.
